BAB IV - Potensi Pengembangan Gut-Brain Axis di Masa Depan
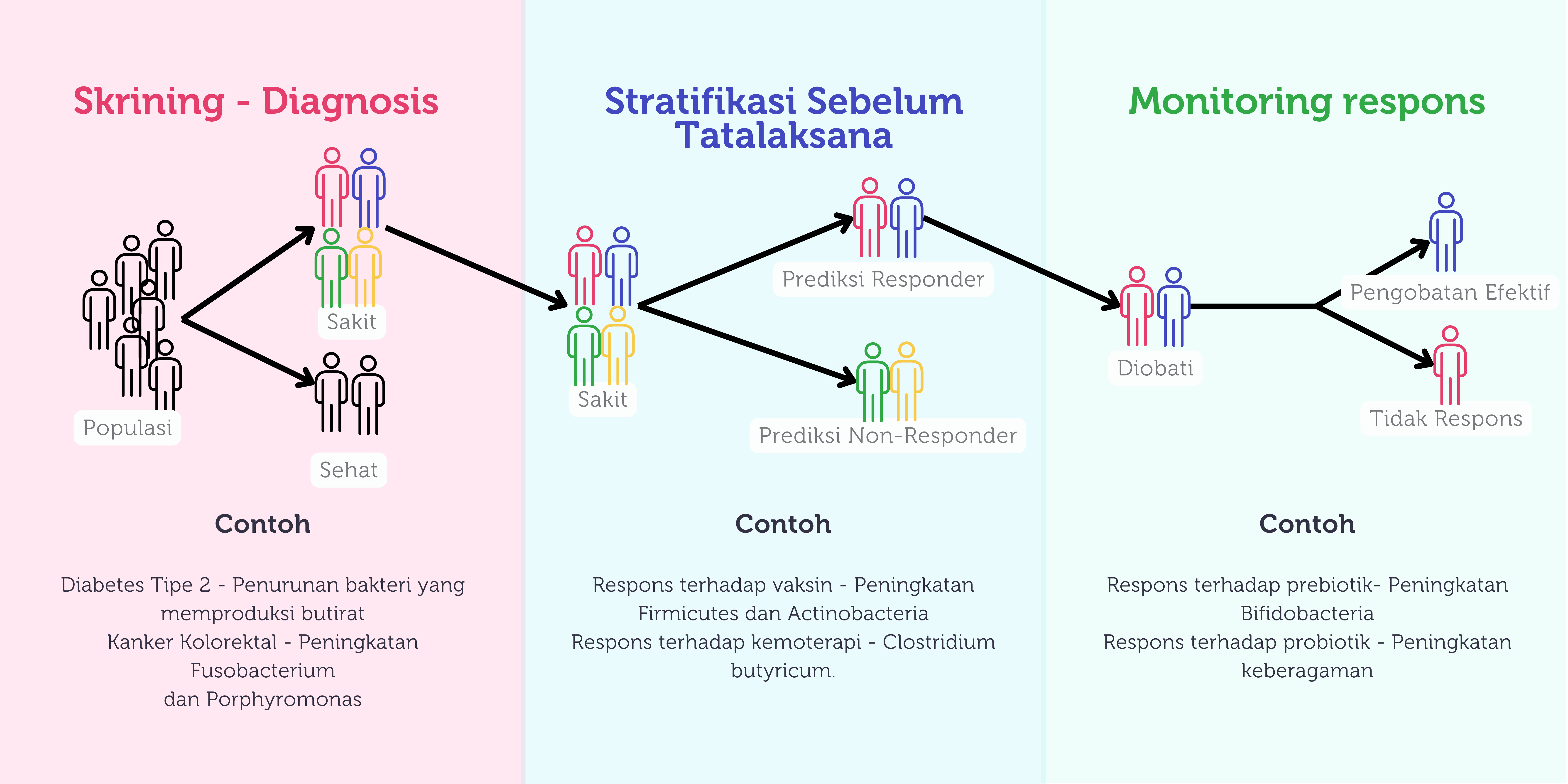 Gambar 4. Potensi pemanfaatan mikrobiota di masa depan (dimodifikasi dari Gulliver et al, 2022).
Gambar 4. Potensi pemanfaatan mikrobiota di masa depan (dimodifikasi dari Gulliver et al, 2022).
Perkembangan pada bidang mikrobiota usus telah membuka peluang untuk lebih memahami hubungan antara sistem imun tubuh, mikrobiota usus, dan gut brain axis. Hal ini juga membuka kesempatan baru bagi peneliti agar dapat menelaah lebih jauh terkait potensi mikrobiota sebagai metode diagnosis, pencegahan serta pengobatan penyakit yang didasari oleh disbiosis.102
Salah satu potensi arah penelitian mikrobiota adalah mengetahui apakah pola mikroba dapat menjadi biomarker untuk mendeteksi suatu penyakit tertentu seperti diabetes tipe 2, kanker kolon, sirosis hati dan karsinoma hepatoseluler. Adanya perubahan atau disbiosis yang spesifik pada setiap jenis penyakit tersebut dapat membantu dalam proses diagnosis.103 Sebagai contoh, pada pasien dengan diabetes tipe 2, ditemukan keberlimpahan yang berkurang pada bakteri penghasil butirat.103
Selain sebagai biomarker, profil mikrobiota usus juga berpotensi memprediksi respons seseorang terhadap tatalaksana yang diberikan. Tidak hanya itu, pemeriksaan mikrobiota juga dapat digunakan sebagai indikator untuk menilai keberhasilan suatu pengobatan. Potensi pemanfaatan mikrobiota dapat dilihat pada Gambar 4.103
Pada akhirnya, melalui pemahaman yang mendalam mengenai profil mikrobiota diharapkan klinisi dapat memberikan pengobatan yang lebih presisi dan spesifik untuk setiap individu sehingga dapat memperbaiki luaran terapi serta meminimalkan efek samping.103
Beberapa strain probiotik seperti Bifidobacterium longum subsp. longum telah terbukti mengurangi stres psikologis yang dirasakan pada orang dewasa sehat. Dalam uji klinis eksploratif, suplementasi dengan Bifidobacterium longum subsp. longum secara signifikan mengurangi stres yang dirasakan dan meningkatkan kualitas tidur. Hal ini menunjukkan bahwa probiotik berpotensi berperan dalam meningkatkan kualitas tidur, yang sering terganggu pada individu dengan depresi dan tingkat stres tinggi.103
Namun, perlu dicatat bahwa temuan ini masih awal dan penelitian lebih lanjut diperlukan untuk memahami sepenuhnya mekanisme di mana probiotik mempengaruhi kesehatan mental serta menetapkan dosis dan strain optimal untuk berbagai kondisi kesehatan mental.104
Pada bidang pediatri, mulai berkembang penelitian terkait peranan mikrobiota usus terhadap efektivitas suatu vaksin.105,106 Beberapa penelitian berhasil membuktikan pada anak dengan diversitas mikrobiota yang lebih tinggi didapatkan adanya respon imun yang lebih baik terhadap vaksin rotavirus, polio, hepatitis B dan vaksin BCG.106 Perubahan jumlah pada jenis bakteri spesifik juga dikaitkan dengan produksi antibodi pada saat vaksinasi. Sebagai contoh, peningkatan keberlimpahan filum Firmicutes dan Actinobacteria dapat berdampak terhadap tingginya produksi antibodi sementara peningkatan keberlimpahan Proteobacteria dan Bacteroidetes berkaitan dengan lebih rendahnya produksi antibodi.107 Hal ini tentu menarik untuk dikembangkan lebih lanjut terutama mengenai pemanfaatan mikrobiota dalam menentukan respons imun terhadap vaksin serta kemungkinan dibutuhkan booster pada kelompok anak tertentu.
Fecal Microbiota Transplant (FMT) merupakan salah satu upaya yang banyak diteliti untuk mengembalikan komposisi dan diversitas mikrobiota. Sejauh ini, keberhasilan FMT telah dibuktikan dapat mengurangi kejadian reinfeksi pada kasus infeksi C.difficile.108 Selain pada saluran cerna, FMT juga sedang diteliti efeknya terhadap resistensi insulin, penyakit liver serta gangguan spektrum autisme.108 Pemberian FMT dikategorikan aman, selama dilakukan skrining yang menyeluruh untuk mencegah adanya transfer multi-drug resistant patogen atau spesies berbahaya lainnya yang dapat mengakibatkan sepsis.108
Ke depan, penting untuk disadari bahwa mikrobiota usus dapat menjadi kontributor utama dalam pencegahan, pengembangan, pengobatan, dan pengelolaan berbagai penyakit, dimana sesuatu yang sederhana seperti pola makan sehari-hari dapat memberikan potensi terapeutik yang signifikan terutama bila dikombinasikan dengan terapi yang ada. Selain itu, dengan meningkatnya pengetahuan tentang mikrobiota usus, dapat pula diciptakan suatu strategi baru dalam pemanfaatan mikrobiota sebagai prediktor prognosis non-invasif dan biomarker untuk berbagai jenis penyakit. Pada akhirnya, pemahaman mengenai mikrobiota usus dapat menjanjikan potensi dan harapan yang menarik untuk membuka peluang baru dalam tatalaksana penyakit yang lebih presisi dan spesifik untuk setiap individu.
Referensi
Appleton J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr Med (Encinitas). 2018;17(4):28-32
Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726.https://doi.org/10.1371/journal.ppat.1003726
Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nature Reviews Neuroscience. 2011;12(8):453-66.https://doi.org/10.1038/nrn3071
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461-78.https://doi.org/10.1038/s41575-019-0157-3
Aresti Sanz J, El Aidy S. Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology (Berl). 2019;236(5):1597-609.https://doi.org/10.1007/s00213-019-05224-0
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nature Microbiology. 2019;4(3):396-403.https://doi.org/10.1038/s41564-018-0307-3
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nature Reviews Microbiology. 2021;19(4):241-55.https://doi.org/10.1038/s41579-020-00460-0
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101(2):246-59.e6.https://doi.org/10.1016/j.neuron.2018.11.018
Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. 2019;216(1):41-59.https://doi.org/10.1084/jem.20180794
Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965-77.https://doi.org/10.1038/nn.4030
Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9(1):233.https://doi.org/10.1038/s41398-019-0570-y
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158.https://doi.org/10.1126/scitranslmed.3009759
Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Metabolism and bacterial pathogenesis. 2015:297-320
Laterza L, Rizzatti G, Gaetani E, Chiusolo P, Gasbarrini A. The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterranean journal of hematology and infectious diseases. 2016;8(1)
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-30
Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475(7356):343-7.https://doi.org/10.1038/nature10244
Schamberger GP, Diez-Gonzalez F. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157: H7. Journal of food protection. 2002;65(9):1381-7
Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543-7
Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, et al. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Applied and environmental microbiology. 2006;72(1):946-9
Khan I, Bai Y, Zha L, Ullah N, Ullah H, Shah SRH, et al. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front Cell Infect Microbiol. 2021;11:716299.https://doi.org/10.3389/fcimb.2021.716299
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1-24.https://doi.org/10.1007/s00394-017-1445-8
Dai Z-L, Li X-L, Xi P-B, Zhang J, Wu G, Zhu W-Y. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino acids. 2013;45:501-12
Hill M. Intestinal flora and endogenous vitamin synthesis. European Journal of Cancer Prevention. 1997;6(2):S43-S5
Reigstad CS, Salmonson CE, Rainey III JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015;29(4):1395
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13(10):701-12.https://doi.org/10.1038/nrn3346
Laue HE, Coker MO, Madan JC. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Frontiers in Pediatrics. 2022;10.https://doi.org/10.3389/fped.2022.815885
Vaher K, Bogaert D, Richardson H, Boardman JP. Microbiome-Gut-Brain Axis in brain development, cognition and behavior during infancy and early childhood. Developmental Review. 2022;66:101038.https://doi.org/https://doi.org/10.1016/j.dr.2022.101038
Laue HE, Coker MO, Madan JC. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Front Pediatr. 2022;10:815885.https://doi.org/10.3389/fped.2022.815885
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiological Reviews. 2019 Oct 1;99(4):1877–2013.
Gao W, Salzwedel AP, Carlson AL, Xia K, Azcarate-Peril MA, Styner MA, et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology. 2019 Jan 2;236(5):1641–51.
Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience. 2018;19(3):123-37
Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon M-C, et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell host & microbe. 2021;29(5):765-76. e3
Callaghan B. Nested sensitive periods: how plasticity across the microbiota-Gut-Brain Axis interacts to affect the development of learning and memory. Current opinion in behavioral sciences. 2020;36:55-62
Cowan CS, Dinan TG, Cryan JF. Annual Research Review: Critical windows–the microbiota–gut–brain axis in neurocognitive development. Journal of Child Psychology and Psychiatry. 2020;61(3):353-71
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762-75
Sordillo JE, Korrick S, Laranjo N, Carey V, Weinstock GM, Gold DR, et al. Association of the infant gut microbiome with early childhood neurodevelopmental outcomes: an ancillary study to the VDAART randomized clinical trial. JAMA network open. 2019;2(3):e190905-e
Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117-21
Reyman M, van Houten MA, van Baarle D, Bosch AA, Man WH, Chu MLJ, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nature communications. 2019;10(1):4997
Rozé J-C, Ancel P-Y, Marchand-Martin L, Rousseau C, Montassier E, Monot C, et al. Assessment of neonatal intensive care unit practices and preterm newborn gut microbiota and 2-year neurodevelopmental outcomes. JAMA network open. 2020;3(9):e2018119-e
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano G, Gasbarrini A, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019 Jan 10;7(1):14.
Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159(3):187-93.https://doi.org/10.1016/j.resmic.2007.12.007
Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, et al. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr. 2018;172(4):368-77.https://doi.org/10.1001/jamapediatrics.2017.5535
Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9(1):4462.https://doi.org/10.1038/s41467-018-06929-0
Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes Metab Syndr Obes. 2012;5:175-89.https://doi.org/10.2147/dmso.S33473
Jiménez E, de Andrés J, Manrique M, Pareja-Tobes P, Tobes R, Martínez-Blanch JF, et al. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J Hum Lact. 2015;31(3):406-15.https://doi.org/10.1177/0890334415585078
Jeong S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clinical and Experimental Pediatrics. 2022;65(9):438
Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins-Browne R, et al. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J Allergy Clin Immunol. 2009;123(2):499-501.https://doi.org/10.1016/j.jaci.2008.11.034
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971-5.https://doi.org/10.1073/pnas.1002601107
MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988.https://doi.org/10.1038/srep08988
Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82.https://doi.org/10.1126/scitranslmed.aad7121
Kim G, Bae J, Kim MJ, Kwon H, Park G, Kim SJ, et al. Delayed Establishment of Gut Microbiota in Infants Delivered by Cesarean Section. Front Microbiol. 2020;11:2099.https://doi.org/10.3389/fmicb.2020.02099
Tirone C, Pezza L, Paladini A, Tana M, Aurilia C, Lio A, et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front Immunol. 2019;10:2910.https://doi.org/10.3389/fimmu.2019.02910
Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69(1):1-10.https://doi.org/10.1016/j.phrs.2012.09.001
Heikkilä MP, Saris PE. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol. 2003;95(3):471-8.https://doi.org/10.1046/j.1365-2672.2003.02002.x
Stsepetova J, Sepp E, Julge K, Vaughan E, Mikelsaar M, de Vos WM. Molecularly assessed shifts of Bifidobacterium ssp. and less diverse microbial communities are characteristic of 5-year-old allergic children. FEMS Immunol Med Microbiol. 2007;51(2):260-9.https://doi.org/10.1111/j.1574-695X.2007.00306.x
Vandenplas Y, Berger B, Carnielli VP, Ksiazyk J, Lagström H, Sanchez Luna M, et al. Human Milk Oligosaccharides: 2'-Fucosyllactose (2'-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients. 2018;10(9).https://doi.org/10.3390/nu10091161
Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15(4):307-10.https://doi.org/10.1038/ni.2847
Francino MP. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front Microbiol. 2015;6:1543.https://doi.org/10.3389/fmicb.2015.01543
Castellani C, Singer G, Kashofer K, Huber-Zeyringer A, Flucher C, Kaiser M, et al. The Influence of Proton Pump Inhibitors on the Fecal Microbiome of Infants with Gastroesophageal Reflux-A Prospective Longitudinal Interventional Study. Front Cell Infect Microbiol. 2017;7:444.https://doi.org/10.3389/fcimb.2017.00444
Levy EI, Hoang DM, Vandenplas Y. The effects of proton pump inhibitors on the microbiome in young children. Acta Paediatr. 2020;109(8):1531-8.https://doi.org/10.1111/apa.15213
Jeong S. Factors influencing development of the infant microbiota: from prenatal period to early infancy. Clin Exp Pediatr. 2022;65(9):439-47.https://doi.org/10.3345/cep.2021.00955
Dubois NE, Gregory KE. Characterizing the Intestinal Microbiome in Infantile Colic: Findings Based on an Integrative Review of the Literature. Biol Res Nurs. 2016;18(3):307-15.https://doi.org/10.1177/1099800415620840
Sung V, D'Amico F, Cabana MD, Chau K, Koren G, Savino F, et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics. 2018;141(1).https://doi.org/10.1542/peds.2017-1811
Rigsbee L, Agans R, Shankar V, Kenche H, Khamis HJ, Michail S, et al. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2012;107(11):1740-51.https://doi.org/10.1038/ajg.2012.287
Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(4):1075-84.https://doi.org/10.3945/ajcn.114.089151
Patole S. Microbiota and Necrotizing Enterocolitis. Psychopharmacology (Berl). 2017 Mar 27;236(5):81–94.
Dobbler PT, Procianoy RS, Mai V, Silveira RC, Corso AL, Rojas BS, et al. Low Microbial Diversity and Abnormal Microbial Succession Is Associated with Necrotizing Enterocolitis in Preterm Infants. Front Microbiol. 2017;8:2243.https://doi.org/10.3389/fmicb.2017.02243
Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011;17(5):557-66.https://doi.org/10.3748/wjg.v17.i5.557
Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14(33):5138-48.https://doi.org/10.3748/wjg.14.5138
Di Costanzo M, De Paulis N, Biasucci G. Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life. 2021 Apr 23;11(5):384.
Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131-48.https://doi.org/10.1111/j.0105-2896.2006.00419.x
Akagawa S, Kaneko K. Gut microbiota and allergic diseases in children. Allergology International. 2022;71(3):301-9.https://doi.org/https://doi.org/10.1016/j.alit.2022.02.004
Cho KY. Association of gut microbiota with obesity in children and adolescents. Clin Exp Pediatr. 2023;66(4):148-54. https://doi.org/10.3345/cep.2021.01837
van Son J, Koekkoek LL, La Fleur SE, Serlie MJ, Nieuwdorp M. The Role of the Gut Microbiota in the Gut–Brain Axis in Obesity: Mechanisms and Future Implications. International Journal of Molecular Sciences. 2021 Mar 15;22(6):2993.
Coomey R, Stowell R, Majewska A, Tropea D. The Role of Microglia in Neurodevelopmental Disorders and their Therapeutics. Curr Top Med Chem. 2020;20(4):272-6.https://doi.org/10.2174/1568026620666200221172619
Zhao H, Zhang H, Liu S, Luo W, Jiang Y, Gao J. Association of Peripheral Blood Levels of Cytokines With Autism Spectrum Disorder: A Meta-Analysis. Front Psychiatry. 2021;12:670200.https://doi.org/10.3389/fpsyt.2021.670200
Lee AS, Azmitia EC, Whitaker-Azmitia PM. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav Immun. 2017;62:193-202.https://doi.org/10.1016/j.bbi.2017.01.019
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. Bmj. 2018;361
Liu F, Li P, Chen M, Luo Y, Prabhakar M, Zheng H, et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Scientific Reports. 2017;7(1):11789.https://doi.org/10.1038/s41598-017-10722-2
Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host & Microbe. 2019;25(6):789-802.e5.https://doi.org/https://doi.org/10.1016/j.chom.2019.05.005
Xu Z, Knight R. Dietary effects on human gut microbiome diversity. British Journal of Nutrition. 2015;113(S1):S1-S5
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11(12):2862
McFarland LV, Evans CT, Goldstein EJC. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2018;5:124.https://doi.org/10.3389/fmed.2018.00124
Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr. 2017;106(5):1274-86.https://doi.org/10.3945/ajcn.117.157529
Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, Tancredi DJ, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr. 2013;163(6):1585-91.e9.https://doi.org/10.1016/j.jpeds.2013.07.017
Smilowitz JT, Moya J, Breck MA, Cook C, Fineberg A, Angkustsiri K, et al. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: a phase I clinical trial. BMC Pediatr. 2017;17(1):133.https://doi.org/10.1186/s12887-017-0886-9
Bi LW, Yan BL, Yang QY, Li MM, Cui HL. Which is the best probiotic treatment strategy to prevent the necrotizing enterocolitis in premature infants: A network meta-analysis revealing the efficacy and safety. Medicine (Baltimore). 2019;98(41):e17521.https://doi.org/10.1097/md.0000000000017521
Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 2012;47(1):241-8.https://doi.org/10.1016/j.jpedsurg.2011.09.064
Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;3(3):Cd005573.https://doi.org/10.1002/14651858.CD005573.pub3
Iheozor-Ejiofor Z, Gordon M, Clegg A, Freeman SC, Gjuladin-Hellon T, MacDonald JK, et al. Interventions for maintenance of surgically induced remission in Crohn's disease: a network meta-analysis. Cochrane Database Syst Rev. 2019;9(9):Cd013210.https://doi.org/10.1002/14651858.CD013210.pub2
Berni Canani R, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow's milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139(6):1906-13.e4.https://doi.org/10.1016/j.jaci.2016.10.050
Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. Isme j. 2016;10(3):742-50.https://doi.org/10.1038/ismej.2015.151
Zhang GQ, Hu HJ, Liu CY, Zhang Q, Shakya S, Li ZY. Probiotics for Prevention of Atopy and Food Hypersensitivity in Early Childhood: A PRISMA-Compliant Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95(8):e2562.https://doi.org/10.1097/md.0000000000002562
Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, Murrell DF, Tang ML, Roberts A, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11(11):Cd006135.https://doi.org/10.1002/14651858.CD006135.pub3
Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. Bmj. 2013;347:f6471.https://doi.org/10.1136/bmj.f6471
Fassio F, Guagnini F. House dust mite-related respiratory allergies and probiotics: a narrative review. Clin Mol Allergy. 2018;16:15.https://doi.org/10.1186/s12948-018-0092-9
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246-59. e6
Beopoulos A, Gea M, Fasano A, Iris F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome–Host Interaction Mechanistic Hypothesis. Nutrients. 2021;14(1):65
Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition. 2019;38(2):522-8
Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448-59. e8
Li Y, Liu T, Qin L, Wu L. Effects of probiotic administration on overweight or obese children: a meta-analysis and systematic review. Journal of Translational Medicine. 2023;21(1):525.https://doi.org/10.1186/s12967-023-04319-9
Ronan V, Yeasin R, Claud EC. Childhood Development and the Microbiome-The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology. 2021;160(2):495-506.https://doi.org/10.1053/j.gastro.2020.08.065
Gulliver EL, Young RB, Chonwerawong M, D'Adamo GL, Thomason T, Widdop JT, et al. Review article: the future of microbiome-based therapeutics. Alimentary Pharmacology & Therapeutics. 2022;56(2):192-208.https://doi.org/https://doi.org/10.1111/apt.17049
Boehme M, Noëla Rémond-Derbez, Lerond C, Lavalle L, Keddani S, Steinmann M, et al. Bifidobacterium longum subsp. longum Reduces Perceived Psychological Stress in Healthy Adults: An Exploratory Clinical Trial. Nutrients. 2023 Jul 13;15(14):3122–2.
Jordan A, Carding SR, Hall LJ. The early-life gut microbiome and vaccine efficacy. The Lancet Microbe. 2022 Oct;3(10):e787–94.
Zimmermann P, Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36(30):4433-9
Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, et al. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134(2):e362-e72
DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. New England Journal of Medicine. 2019;381(21):2043-50.https://doi.org/10.1056/NEJMoa1910437
If you liked this post you may also like