Microbiome and Infection: A Case for “Selective Depletion”
Key insights
Microbial infections can be recognized as a disruption of the normal homeostatic relationship between a particular microbe and the host. There are many instances where the pathogen is not acquired but is already a member of the microbiome, e.g., Clostridioides difficile, Staphylococcus aureus, or certain strains of Escherichia coli. Often, these organisms only cause disease when the surrounding microbiome is compromised, enabling the pathogen to grow and result in disease symptoms in the host. Thus, the microbiome itself should be treated as a virtual organ and protected during therapeutic interventions. A targeted approach towards eliminating unwanted microbial pathogens is termed “selective depletion,” whereby pathogen numbers are curtailed by a narrow-spectrum inhibitor, but the microbiome is protected and can thus play a role in restoring health and suppressing pathogen outgrowth in the infected patient.
Current knowledge
From birth to adulthood, all our exposed bodily surfaces become colonized by thousands of different microbial strains. The human body consists of hundreds of micro-environments that facilitate the growth of various microbes (the microbiome). In turn, the microbiome fulfills many functions that are critical for human health. Under a normal state of homeostasis, the microbiome acts as a “virtual organ,” constantly signaling to the digestive, immune, nervous, and endocrine systems in a process that maintains health and prevents disease. Infection can be viewed as any interaction between a microbe and the host that results in damage severe enough to be manifested in the form of disease symptoms.
Practical implications
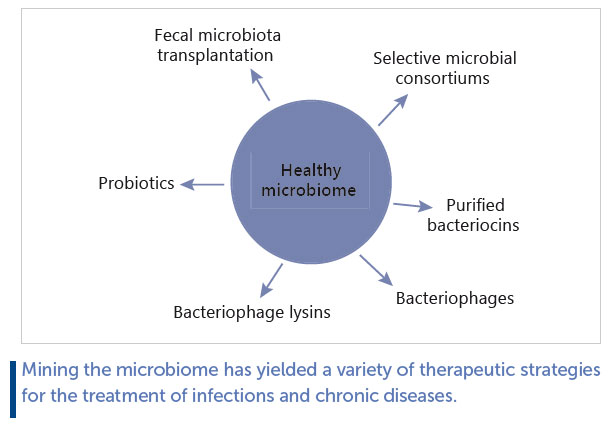
What is it about the microbiome that keeps pathogens in check, and can we harness the microbiome itself for selective therapies against specific pathogens? Microbiome-derived anti-infection strategies have ranged from using an entire intact microbiome, a selected microbial consortium, or single biological entities. Recent advances in mining the microbiome have taken this one step further. A targeted approach involves the isolation and purification of bacteriocins, toxins produced by bacteria that inhibit the growth of related bacterial strains. For example, thuricin CD is produced by a strain of Bacillus thuringiensis and is a very narrow-spectrum bacteriocin targeting C. difficile. This approach paves the way for selective depletion of pathogens without collateral damage to the microbiome.
Recommended reading
De Maesschalck V, Gutiérrez D, Paeshuyse J, Lavigne R, Briers Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit Rev Microbiol. 2020;46:548–64.
Key Messages
• The microbiome is a virtual organ that should be protected in therapeutic interventions.
• Narrow-spectrum inhibitors can “selectively deplete” the pathogen while protecting the microbiome.
• The microbiome itself can be a valuable source of antimicrobials. Keywords Bacteriocin · Bacteriophage · Probiotics · Infection · Microbiome
Abstract
In most instances where a pathogen has initiated an infection, the primary goal of the treating physician or pharmacist is to eliminate the pathogen. In the absence of knowledge of the precise identity of the problem-causing microbe, a broad-spectrum antimicrobial gives the best chance of success. This approach has saved many lives and is an invaluable tool in fighting infections. However, perhaps our current appreciation of the importance of the microbiome in human health should give us pause. We can regard the microbiome as a virtual organ within the human body, and we would surely hesitate to advance any therapeutic approach that would cause substantial damage to one of our organs. This is one consequence of many broad-spectrum antimicrobial therapies. There may be instances where a more precise approach would be useful. I have termed this “selective depletion”; a concept where pathogen numbers are curtailed by a narrow-spectrum inhibitor but the microbiome is protected and can play a role in restoring health and suppressing the outgrowth of the pathogen in the infected patient. It may well be that the best reservoir of microbiome-friendly antimicrobial agents is the microbiome itself, and I provide examples of where the microbiome has been mined for novel precision antimicrobials.
Microbiome, Pathogens, and Host
As a rule, microbes are good for us. We interact with trillions of microbes every day, and almost all these interactions are mutually beneficial, or at the very least are benign. Our most important microbial interaction is that between us and our microbiome, our fellow travellers on our journey through life.
We are born into a microbial world, and as adults, all our exposed surfaces are colonised with thousands of microbial strains selected from the millions we have encountered since birth [1] . We provide our microbes with a somewhat protected, albeit highly competitive, environment. It is more correct to state that we provide hundreds of micro-environments or niches. We as hosts provide sustenance and permissive environmental conditions, while our microbial partners fulfil many functions important to human health. We share the same chemical language, and so there is constant communication between our microbes and our nervous system, our immune systems, and our mucosal surfaces. In a healthy human, these interactions with our microbial “virtual organ” [2] is important for maintaining health and preventing disease. The microbiome is a valuable partner and, as with any other organ, should be protected insofar as possible.
In this context, we can see microbial infections as an aberration where a specific microbe has broken with the normally benign or beneficial relationship between microbes and hosts. We call these microbes pathogens or opportunistic pathogens. Infection describes any interaction between a microbe and a host that results in damage that is severe enough to be manifested in the form of symptoms. Infection usually follows a very prescribed path, initiated by acquisition or exposure to the infectious microbe (pathogen), engagement between the pathogen and the host in terms of survival, colonisation, growth, and ultimately damage mediated by virulence factors such as invasive mechanisms and/or the production of toxins. The damage caused can result in mild symptoms or death depending on a myriad of factors. These factors include the identity of the pathogen, including the genus, species, and even the genetic complement of a given strain; the possession and expression of virulence factors; the dose; the circumstances surrounding the exposure in terms of food carriers or breach of barriers (skin or mucosal surface); the genetics of the host; the hosts immune status; and of course, the commensal microbes present at the site of infection (the microbiome).
There are many instances where the pathogen is not acquired but is already a member of our microbiome, for example, Clostridioides difficile , Staphylococcus aureus , or certain strains of Escherichia coli . These pathogens often only cause disease when the surrounding microbiome is compromised in some way, and the pathogen manages to grow and use its virulence factors to cause damage to the host. We can regard the microbiome as a barrier to infection ( Fig. 1 ). Antibiotic treatment can be an effective solution to reduce or even eliminate a pathogen but can also lead to further damage to the microbiome. For C. difficile , this can result in recurring bouts of diarrhoea and even fatal outcomes. Most microbiomes will recover from antibiotic exposure and will revert to a level of complexity and community structure that approximates the pre-treatment microbiome, but of course, there will also be an undesirable selection pressure for the emergence of antibiotic resistance in both target and nontarget species. A link between microbiome composition and sensitivity to viral infection in humans has also been reported recently [3] .
What is it about the microbiome that keeps these pathogens in check, and can we develop effective therapies aimed at selectively depleting the pathogen during an infection? I use the term “selective depletion” to describe any treatment that causes minimal damage to the microbiome but reduces the pathogen to levels that do not lead to symptoms or cause long-term damage to the host. What kind of strategies would meet this objective of selective depletion? Perhaps the best place to start is the microbiome itself. Can we explore the microbiome for strategies that can prevent and even treat infections? I will draw upon some of our own work in this area to illustrate some of the possibilities of “mining the microbiome” for novel anti-infectives.
Mining the Microbiome
Microbiome-derived anti-infection strategies have ranged from using an entire intact microbiome or a microbial consortium to selecting single biological entities (bacteria, fungi, bacteriophage, or bacterial metabolites) ( Fig. 2 ). In most instances, the objective is one of selective depletion, rather than the broad-spectrum approach typical of many antibiotic or antiviral strategies. Much of the data generated to date have been acquired from animal models since it can be difficult to design and perform experiments on humans with respect to infection.
Faecal Microbiota Transplantation
Faecal microbiota transplantation (FMT) is perhaps the most dramatic example of mining the microbiome for solutions to infections. A recent systematic review and meta-analysis concluded that there is high-quality evidence that FMT is effective in breaking the vicious cycle of recurring C. difficile infections [4] , although a similar success rate has not been reported for non-infectious diseases such as irritable bowel syndrome [5] . Essentially, FMT involves collecting the entire microbiome from one individual in the form of a faecal donation and transplanting it with minimal processing to the colon of a patient with recurring C. difficile infections. The generally accepted opinion is that FMT “repairs” a microbiome that has been damaged by the antibiotic therapies used to/try to control the initial infection(s) and allows the recipient to use this more diverse microbiome to suppress subsequent outgrowth of the pathogen. Another strategy linked to FMT is using an undefined microbial consortium composed of spores purified from a faecal sample and transferred to a patient with a similar objective of restoring diversity and suppressing pathogen-induced damage. This intervention had less successful results in published phase 2 clinical trials [6] but has the potential to be the platform for a range of microbiome-based intervention strategies.
Probiotics
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [7] . There is evidence that probiotics can be used to prevent and treat infection, and some of these data have been generated in humans. The strength of the data is not entirely consistent, since most meta-analyses and systematic reviews are performed across a range of different probiotic interventions, dose ranges, and disease outcomes. Nonetheless, there have been positive outcomes. A recent meta-analysis on the ability of probiotics to prevent necrotizing enterocolitis in preterm neonates concluded that there are “significant benefits of probiotic supplements in reducing death and disease in preterm neonates” [8] . A similar analysis for the use of probiotics in upper respiratory tract infections concluded that “low-quality evidence provides support that probiotics have potential efficacy for preventing upper respiratory tract infection episodes in adults” [9] . Yet another meta-analysis conducted on the use of probiotics in children with acute diarrhoea “ supports the potential beneficial roles of probiotics and synbiotics for acute diarrhoea in children” [10] . There are also single clinical trials with impressive evidence, for example, a randomised clinical trial in India involving over 45,000 children using a symbiotic composed of Lactiplantibacillus plantarum together with fructooligosaccharides reported a significant reduction in sepsis and death in the treatment cohort [11] .
Mechanistic insights have also been generated in animal models. We were able to demonstrate that bacteriocin production is responsible for the protective effect of Ligilactobacillus salivarius UCC118 against deliberate infection of mice with Listeria monocytogenes [12] . We showed that the bacteriocin was highly active against L. monocytogenes and that a non-bacteriocin-producing genetic knockout had no protective effect against the pathogen in the same model. Other anti-infective mechanisms could include interacting with the immune system or improving barrier function [13] . Bacteriocins represent another example of the “selective depletion” concept in that they are usually narrow spectrum and should be rapidly broken down by proteases and peptidases in the gut and therefore unlikely to select for resistance.
Bacteriocins
Concentrated or purified bacteriocins have also been explored for their potential in preventing or treating infection (reviewed by [14–16] ). Bacteriocins have been largely tested in laboratory animal models, although a broad-spectrum lantibiotic, nisin, has been used commercially to prevent mastitis in dairy cattle [17, 18] . Some bacteriocins such as nisin are relatively broad spectrum, but an attempt has also been made to use narrow-spectrum bacteriocins for “selective depletion” of specific pathogens. One example is the discovery of thuricin CD, a very narrow-spectrum bacteriocin targeting C. difficile [19, 20] . Thuricin CD is produced by a strain of Bacillus thuringiensis that was mined from the human gut microbiome in an extensive screening program. It was subsequently demonstrated in improvised models of the human colon (faecal fermentations) that thuricin CD could dramatically reduce C. difficile levels in a highly complex microbiome without causing substantial collateral damage to the other members of the community. Antibiotics or broad-spectrum bacteriocins deployed in the same model also reduced levels of C. difficile but also caused significant shifts in microbiome composition [20] . While this approach has not been validated in humans or animals, it provides a glimpse of future developments in this exciting area.
Bacteriocins also have a potential as antimicrobials for treating skin infections, some as broad-spectrum inhibitors such as garvicin KS and micrococcin P1 [21, 22] , but others as potentially narrow-spectrum inhibitors that could be used in selective depletion strategies. Because bacteriocins are gene encoded, they can be engineered to improve their physicochemical characteristics and/or inhibition spectrum [23] . Bacteriocins can also be used in combination with other antimicrobials for greater effect [24] .
Bacteriophage and Bacteriophage Lysins
Bacteriophages (phages) are bacterial viruses that often have a very narrow-spectrum of inhibition. There are several advantages and disadvantages to using bacteriocins to treat infections, a concept often referred to as phage therapy [25] . Disadvantages include the narrow host ranges, although, of course, this could be regarded as an advantage in selective depletion strategies. This disadvantage can be addressed by using cocktails of phages to cover a wider spectrum of target bacteria, or by carefully selecting the correct phage from an existing phage bank to use against a specific pathogenic strain infecting a patient. Another disadvantage is the ease with which bacteria can develop resistance to the phage attack, again an issue that may be overcome by using multiple phages in cocktails. Advantages include their widespread distribution in nature and subsequent ease of isolation in many instances, and, of course, their ability to multiply at the site of infection. However, the clinical evidence is scarce. Phages have been used for decades in eastern Europe, and it is logical to assume they must have benefits in that they have survived as frontline treatments for so many years [26] . In the West, phages have been used in some high-profile instances in single patients with infections that were recalcitrant to antibiotic therapies [27, 28] . Phages can potentially be applied on the skin, by inhalation, or by ingestion [29] .
Lysins are enzymes produced by bacteriophages that are responsible for lysing the target bacterium following multiplication of phage particles. Phage lysins target the cell wall and can also act from outside the cell. Lysins can be broader spectrum than their carrying phages, but are still limited to the species or genus level, making them good candidates for selective depletion strategies. There have been many attempts to use phage lysins to target specific pathogens (reviewed by [30, 31] , particularly on the skin [32] and in the respiratory tract [33] . As is the case for bacteriocins, these gene-encoded antimicrobials can readily be engineered for greater efficacy [34] .
Conclusions
The microbiome provides both a barrier to infection and a source that can be mined for novel antimicrobials. While these antimicrobials, including FMT, microbial consortia, probiotics, bacteriocins, and bacteriophages (and their lysins), have often been analysed for their solo impact on infection, there is no reason why these could not be used as combinatorial treatments. Perhaps the most attractive feature of these microbiome-based inhibitors is their potential narrow spectrum of inhibition that would act to selectively deplete the pathogen, while preserving the complexity of the surrounding microbiome. This might require precise identification of the pathogen before initiating treatment, but this is not a significant problem with chronic infections and is becoming less of a problem with speedy high-throughput bacterial identification becoming more accessible for acute infections [35] . The future of smart antimicrobial therapies may not involve pathogen elimination with collateral damage to the microbiome (broad-spectrum approaches) but may well lie in restricting the growth of pathogens to a point where they cannot cause disease while harnessing the power of the microbiome to ensure a return to a healthy state – a strategy I designate as selective depletion.
Statements of Ethics
No ethical approval is required as there is no original data in this article.
Conflict of Interest Statement
The writing of this article was supported by Nestlé Nutrition Institute and the author declares no other conflicts of interest.
References
1 Hill C. You have the microbiome you deserve. Gut Microb . 2020;1: E3.
2 O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep . 2006; 7: 688–93.
3 Patin NV, Peña-Gonzalez A, Hatt JK, Moe C, Kirby A, Konstantinidis KT. The role of the gut microbiome in resisting norovirus infection as revealed by a human challenge study. mBio . 2020; 11: e02634–20.
4 Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine . 2020; 29–30: 100642.
5 Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019; 114: 1043–50.
6 McGovern BH, Ford CB, Henn MR, Pardi DS, Khanna S, Hohmann EL, et al. SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis . 2020; ciaa387.
7 Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol . 2014; 11(8): 506–14.
8 Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics . 2010; 125: 921–30.
9 Li L, Hong K, Sun Q, Xiao H, Lai L, Ming M, et al. Probiotics for preventing upper respiratory tract infections in adults: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med . 2020; 2020: 8734140.
10 Yang B, Lu P, Li M-X, Cai X-L, Xiong W-Y, Hou H-J, et al. A metaanalysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine . 2019; 98: e16618.
11 Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature . 2017; 548(7668): 407–12.
12 Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A . 2007; 104(18): 7617–21.
13 Preidis GA, Hill C, Guerrant RL, Ramakrishna BS, Tannock GW, Versalovic J. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology . 2011; 140(1): 8–14.
14 Lopetuso LR, Giorgio ME, Saviano A, Scaldaferri F, Gasbarrini A,Cammarota G. Bacteriocins and bacteriophages: therapeutic weapons for gastrointestinal diseases? Int J Mol Sci . 2019; 20(1): 183.
15 Meade E, Slattery MA, Garvey M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: resistance is futile? Antibiotics . 2020; 9(1): 32.
16 Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, et al. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev . 2021; 45(1): fuaa039.
17 Cao LT, Wu JQ, Xie F, Hu SH, Mo Y. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci . 2007; 90(8): 3980–5.
18 Halasa T, Huijps K, Østerås O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q . 2007; 29(1): 18–31.
19 Rea MC, Sit CS, Clayton E, O’Connor PM, Whittal RM, Zheng J, et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A . 2010; 107(20): 9352–7.
20 Rea MC, Dobson A, O’Sullivan O, Crispie F, Fouhy F, Cotter PD, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium
difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A . 2011; 108(Suppl 1): 4639–44.
21 Kranjec C, Ovchinnikov KV, Grønseth T, Ebineshan K, Srikantam A, Diep DB. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes . 2020; 6(1): 58.
22 Ovchinnikov KV, Kranjec C, Thorstensen T, Carlsen H, Diep DB. Successful development of bacteriocins into therapeutic formulation for treatment of MRSA skin infection in a murine model. Antimicrob Agents Chemother . 2020; 64(12): e00829–20.
23 Field D, Cotter PD, Hill C, Ross RP. Bioengineering lantibiotics for therapeutic success. Front Microbiol . 2015; 6: 1363.
24 Todorov SD, de Paula OAL, Camargo AC, Lopes DA, Nero LA. Combined effect of bacteriocin produced by Lactobacillus plantarum ST8SH and vancomycin, propolis or EDTA for controlling biofilm development by Listeria monocytogenes. Rev Argent Microbiol. 2018; 50(1): 48–55.
25 Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol . 2019; 10: 513.
26 Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Med Mal Infect . 2008; 38(8): 426–30.
27 Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med . 2019; 25(5): 730–3.
28 Strathdee SA, Patterson TL, Barker T. The perfect predator: a scientist’s race to save her husband from a deadly superbug . 1st ed. New York ; Boston: Hachette Books; 2019.
29 Loh B, Gondil VS, Manohar P, Khan FM, Yang H, Leptihn S. Encapsulation and delivery of therapeutic phages. Appl Environ Microbiol AEM . Forthcoming 2020.
30 Vázquez R, García E, García P. Phage lysins for fighting bacterial respiratory infections: a new generation of antimicrobials. Front Immunol . 2018; 9: 2252.
31 Fischetti V. Development of phage lysins as novel therapeutics: a historical perspective. Viruses . 2018; 10: 310.
32 Wang Z, Kong L, Liu Y, Fu Q, Cui Z, Wang J, et al. A phage lysin fused to a cell-penetrating peptide kills intracellular methicillinresistant Staphylococcus aureus in keratinocytes and has potential as a treatment for skin infections in mice. Appl Environ Microbiol. 2018; 84(12): e00380.
33 Wienhold S-M, Lienau J, Witzenrath M. Towards inhaled phage therapy in Western Europe. Viruses . 2019; 11: 295.
34 De Maesschalck V, Gutiérrez D, Paeshuyse J, Lavigne R, Briers Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit Rev Microbiol . 2020; 46: 548–64.
35 Özenci V, Rossolini GM. Rapid microbial identification and antimicrobial susceptibility testing to drive better patient care: an evolving scenario. J Antimicrob Chemother . 2019; 74: i2–5.